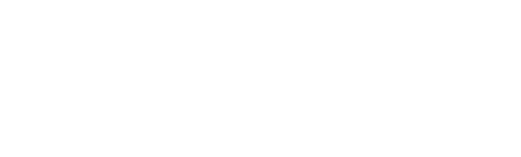Hemoglobin Dissociation Curve Shift
Read the case study below and answer the questions.
Deliverables
- With respect to hemoglobin loading, please explain the relationship between binding of oxygen (O2) and carbon monoxide (CO) to the hemoglobin molecules.
- During the ambulance ride, a pulse oximeter showed 100% O2 saturation. Why is that different from the 72% measured at the hospital?
- One course of treatment is a hyperbaric oxygen treatment. How does a hyperbaric chamber work?
- Adams blood work shows him to be in an acidosis (normal blood pH is 7.35-7.45). Explain how this will shift the hemoglobin dissociation curve and why.
Adam and his family decided to take a trip to the mountains for the weekend in late February. They had a small cabin and looked forward to a weekend away from the big city. The family had a wonderful time together on Saturday morning hiking in the woods and enjoying nature. However, Saturday afternoon a storm rolled in bringing snow and subfreezing temperatures.
Since the heater in the cabin wasn’t working well, Adam’s mother and sister decided to drive into the nearest town to spend the night. Adam and his father, not being sissies, stayed at the cabin where they started a gas heater to keep them warm.
The next morning Adam’s mother and sister returned to find both Adam and his father unconscious. An ambulance was called and they were both transported to the nearest hospital. Adam had arterial blood gases drawn with the following results:
- pH 7.2
- PaCO2 31.4,
- PaO2 40.7 mmHg
His oxygen saturation was 72%. Adam was diagnosed with carbon monoxide poisoning.
Question one
The relationship between the binding of oxygen (O2) and carbon monoxide (CO) to hemoglobin molecules is based on their affinity for the heme groups in hemoglobin. Hemoglobin has four heme groups, each capable of binding to one molecule of either oxygen or carbon monoxide. Oxygen binds to hemoglobin in a cooperative manner. When the first oxygen molecule binds to a heme group, it induces a conformational change in the hemoglobin molecule, making it easier for subsequent oxygen molecules to bind. This cooperative binding allows hemoglobin to efficiently pick up oxygen in the lungs where oxygen levels are high and release it in tissues where oxygen levels are low.
Besides, carbon monoxide has a much higher affinity for hemoglobin than oxygen. Carbon monoxide binds to the heme group of hemoglobin more tightly and forms a stable complex called carboxyhemoglobin. This binding is reversible but significantly stronger than the binding of oxygen. Once carbon monoxide binds to a heme group, it reduces the oxygen-carrying capacity of hemoglobin by displacing oxygen molecules. The affinity of carbon monoxide for hemoglobin is approximately 200-250 times greater than that of oxygen. As a result, even low levels of carbon monoxide can displace a significant amount of oxygen from hemoglobin, leading to reduced oxygen delivery to tissues and carbon monoxide poisoning.